Why Does Sulfur Dioxide Have a Low Boiling Point
Sulfur dioxide has a. The bond energy of Si is generally considered to be lower than that of the C-C so a simple explanation is that diamond has a stronger bond.
Why does Sulphur hexafluoride has a low boiling point.
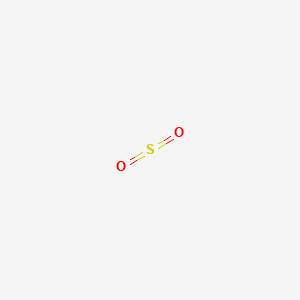
. Sulfur dioxide irritates the respiratory tract and increases the risk of tract infections. This is why simple molecular substances such as carbon dioxide have a low boiling point. Sulfur dioxide IUPAC-recommended spelling or sulphur dioxide traditional Commonwealth English is the chemical compound with the formula S O 2It is a toxic gas responsible for the smell of burnt matchesIt is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur-bearing fossil fuels.
Wiki User 2011-02-20 233902. Very toxic by inhalation and may irritate the eyes and mucous membranes. Explain why are PVC softens and melts when heated.
However it has low boiling point because each individual molecule is joined together by weak intermolecular forces. Carbon dioxide is a simple molecular gas. Subsequently question is does silicon dioxide have a high melting point.
Short-term exposures to SO 2 can harm the human respiratory system and make breathing difficult. Explain why a mixture of CHCl3 and propanone can have a higher boiling point measured under the same atmospheric conditions than either of the. Answered by Alex M.
Why is sulfur dioxide bad for humans. Like other covalent substances there are strong covalent bonds within the molecules but only weak Van der Waals forces between molecules so that it has a low boiling point and melting point actually it sublimes at -637C. Under prolonged exposure to fire or heat the containers may rupture violently and rocket.
When sulfur dioxide is boiled it is the weak intermolecular forces which are broken and not the strong covalent bonds. Sulfur dioxide appears as a colorless gas with a choking or suffocating odor. What are the health effects of SO 2.
It causes coughing mucus secretion and aggravates conditions such as asthma and chronic bronchitis. 416 understand how sulfur dioxide and oxides of nitrogen oxides contribute to acid rain. As sulfur is a non-metal it exhibits these properties and this is why its melting point is rather low compared to other substances particularly metals.
People with asthma particularly children are sensitive to these effects of SO 2. It consists of a carbon atom covalently bonded to two oxygen atoms. Health effects Sulfur dioxide affects the respiratory system particularly lung function and can irritate the eyes.
This makes diamonds melting point and boiling point very high. 3 marks Small molecules with weak intermolecular forces so only a small amount of energy is needed. Propanone CH3COCH3 has a boiling point of 562ºC.
Carbon dioxide chemical formula CO 2 is a chemical compound occurring as an acidic colorless gas with a density about 53 higher than that of dry air. Why is silicon dioxide melting point lower than diamond. Why does Sulphur dioxide have a low boiling point.
Does nitrogen dioxide have a low melting and boiling point. It occurs naturally in Earths atmosphere as a trace gasThe current concentration is about 004 412 ppm by volume. 2 marks Hard and unreactive.
Answer 1 of 4. For an exam answer you would need to state that sulfur dioxide is a small molecule 1 mark so it has weak intermolecular forces between molecules 1 mark therefore only a small amount of energy is required to seperate the molecules of sulfur dioxide 1 mark so it has a low boiling point. Give two reasons why alloys are used in ballpoint pens.
Nitrogen Dioxide boils at 21 C which is low. Explain why sulfur dioxide has a low boiling point. Click to see full answer.
SO 2 emissions that lead to high concentrations of SO 2 in the air generally also lead to the formation of other sulfur oxides. It melts at -112 C which leaves little. Only a small of amount of energy is required to break the intermolecular forces so the boling point is low.
Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. Explain why silicon dioxide has a high melting point above 1600ºC whereas carbon dioxide is a gas at room temp and pressure. So when carbon dioxide changes from a solid to a gas for example that process can be represented as.
Sulfur dioxide is a simple molecule.
Why Are The Intermolecular Forces Of Sulphur Dioxide Greater Than Carbon Dioxide Quora
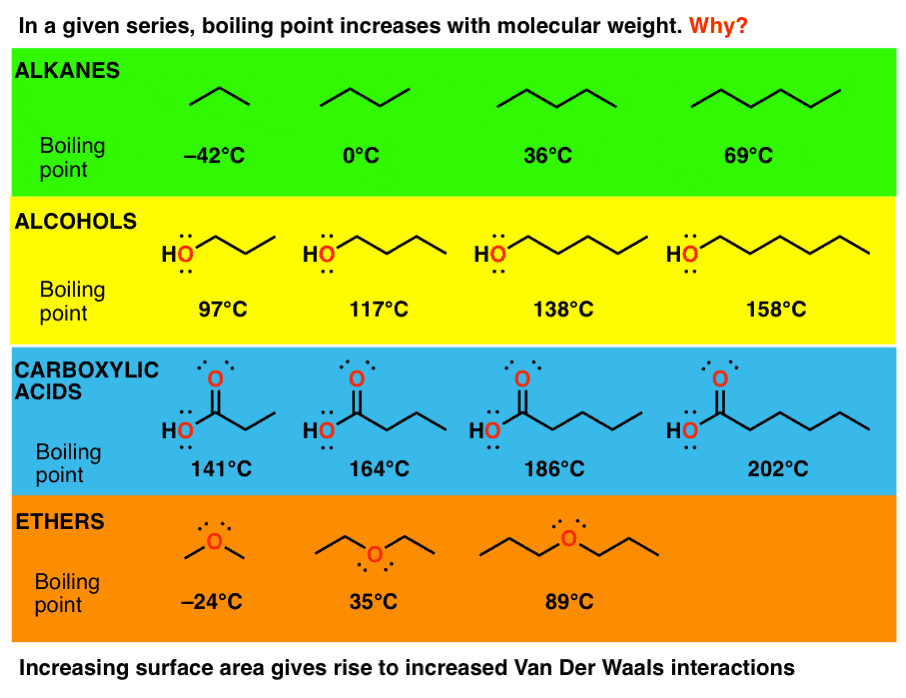
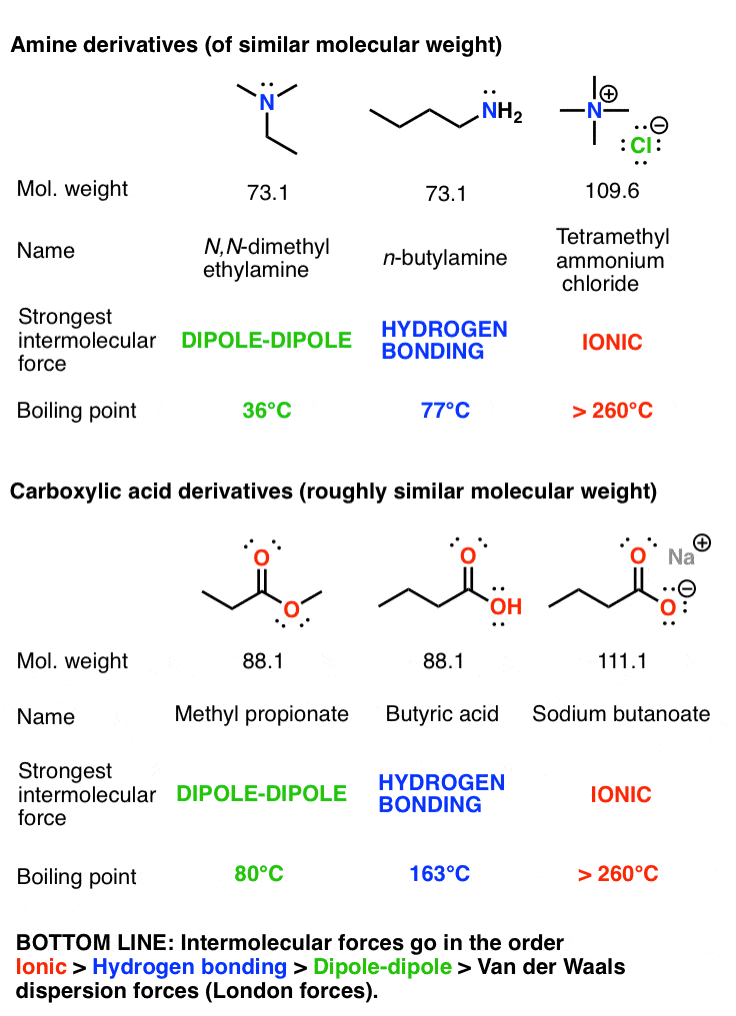
3 Trends That Affect Boiling Points Master Organic Chemistry

3 Trends That Affect Boiling Points Master Organic Chemistry

3 Trends That Affect Boiling Points Master Organic Chemistry
Comments
Post a Comment